COMSOL has the best multiphysical simulation capabilities in my experience. Technical support from Elisa at TECHNIC as well as the engineers at COMSOL has been great.
COMSOL is an important part of our research in plasma physics. We use it in the design of plasma systems and it helps us to obtain a greater understanding of the underlying physics. We have always valued the quick support from TECHNIC and COMSOL and it has been a pleasure to work with them.
Comsol has become a valuable part of our design and decision making process. The exceptional flexibility and access to the physics and solvers in Comsol has allowed us to have deeper understanding on thermomechanical solutions. Technic and Comsol have always been quick and helpful to resolve any issues and provide helpful advice on their products.
At Scion we use COMSOL Multiphysics to understand energy processes, such as the interplay of non-linear solid mechanics and heat & mass transfer during biomass compaction, to design new or more efficient processes.
We use COMSOL Multiphysics to design the customised muffler. With it, we can simulate the insertion loss at different spectrum with different muffler designs.
The Fuel Cell & Electrolyser Module is an add-on to the COMSOL Multiphysics software for gaining a deeper understanding of fuel cell and electrolyzer systems, which is useful for designing and optimising the electrochemical cells. The types of systems that may be studied include proton exchange membrane fuel cells (PEMFCs), hydroxide exchange (alkaline) fuel cells (AFCs), molten carbonate fuel cells (MCFCs), and solid oxide fuel cells (SOFCs), as well as the corresponding water electrolyser systems. However, the module accommodates all types of fuel cells and electrolysers.
As with every product in the COMSOL product suite, multiphysics capabilities are built into the software for including multiphase fluid flow, heat transfer, thermodynamics properties, and more.
The Fuel Cell & Electrolyser Module features predefined formulations for the most common types of hydrogen fuel cell, accounting for the electrodes, electrolyte, as well as current collectors and feeders. Examples of fuel cell types that can be modelled are the PEMFC, AFC, PAFC, SOFC, MCFC, and high-temperature PEMFC, to mention a few.
Modelling and simulation can be used to predict the current and potential distribution, the chemical species distribution, and the temperature distribution in a fuel cell. In this way, the cell can then be designed for the best possible utilisation and operation for a given set of conditions. Important aspects are the removal of water and avoiding nonuniform utilisation of the cell, which may result in poor performance and decreased lifespan. Additionally, microscopic aspects of the gas diffusion electrodes and the active layers can be studied, such as catalyst loading, particle size distribution, and bimodal pore distribution.
Analyses cover 0D, 1D, 2D, and 3D during transient or steady-state operation. The physics-based models can also be formulated in the frequency domain to simulate impedance spectroscopy experiments.
The lowered costs for wind and solar power has led to a larger production of electricity from electrolyser sources. This also means that there is a larger surplus of electricity when the wind blows and the sun shines. Storing this electricity in batteries is expensive. Instead, electrolysers can be used to produce hydrogen from electricity through water electrolysis.
The design of a water electrolyser is similar to that of a hydrogen fuel cell, with the difference being that in comparison to a fuel cell, the current runs in the reverse direction, the cathode is the negative electrode, and the anode is the positive electrode. The models within the Fuel Cell & Electrolyser Module involve the description of the gas diffusion electrodes, the active layer, the electrolyte separator, and the bipolar plates with the channels. Models can be defined at the unit cell as well as the stack level.
As with the fuel cells, the models are physics-based and cover 0D, 1D, 2D, and 3D, for steady-state and transient operations.
The functionality of the Fuel Cell & Electrolyser Module is not limited to water electrolysers. Any electrochemical cell or electrolyser can be modelled. This includes the ability to describe gas evolution and laminar multiphase flow. For systems such as chlorate electrolysis and the chlor-alkali membrane process, the module can be combined with the CFD Module to also treat turbulent flows.
The Fuel Cell & Electrolyser Module contains detailed descriptions of the transport equations, chemical reactions, and electrochemical reactions that occur in fuel cells and electrolysers. The software formulates the model equations for gas diffusion electrodes, pore electrolyte, electrolyte (separator), and bipolar plates, which are the main components in these systems. This implies modelling the cells in 0D, 1D, 2D, or full 3D during stationary operation, or for full transient experiments and operation.
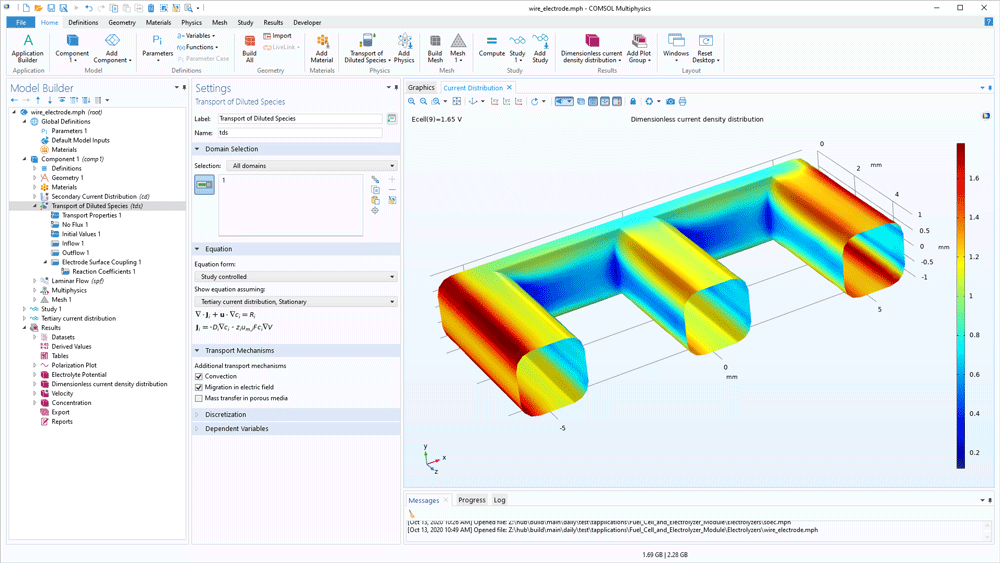
Modelling gas diffusion electrodes (GDEs) in the Fuel Cell & Electrolyser Module is very straightforward. The transport equations in the gas phase and in the pore electrolyte are automatically defined in the user interface based on the boundary conditions added. The software contains separate domain features for defining the hydrogen and oxygen electrodes. The main electrode reactions are predefined, but you can change the kinetics and add bi- and parasitic reactions.
The transport of species in the gas phase is automatically coupled with the transport in the gas channels. The fluid flow is defined for the gas channel and for the porous structure using the Brinkmann equations to model fully coupled free and porous media flow.
The charge balance in the electrolyte (separator) and the pore electrolyte (the electrolyte in the active layer or in the GDE) are also defined. They are automatically coupled to the transport equations in the gas phase through the electrochemical reactions and Faraday's law.
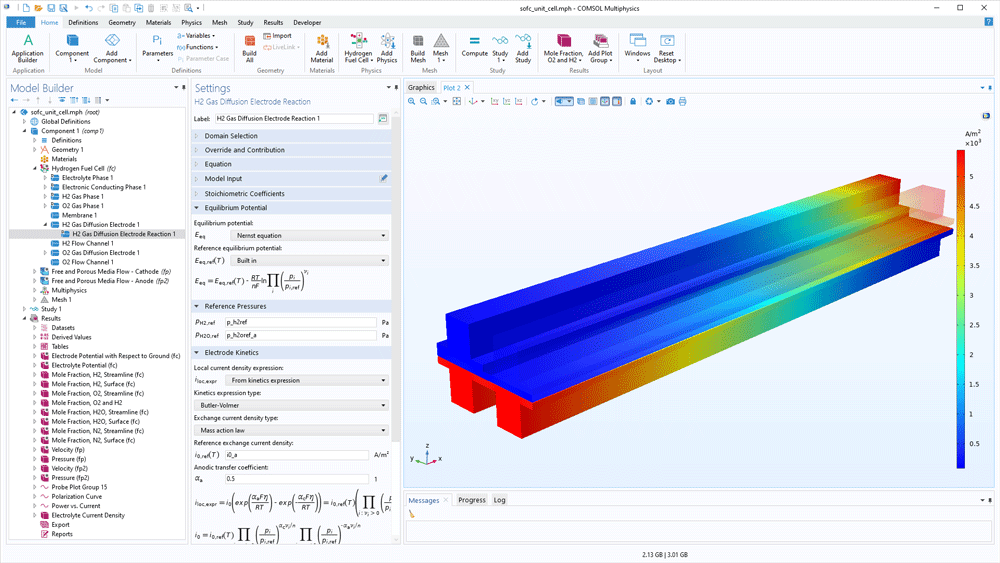
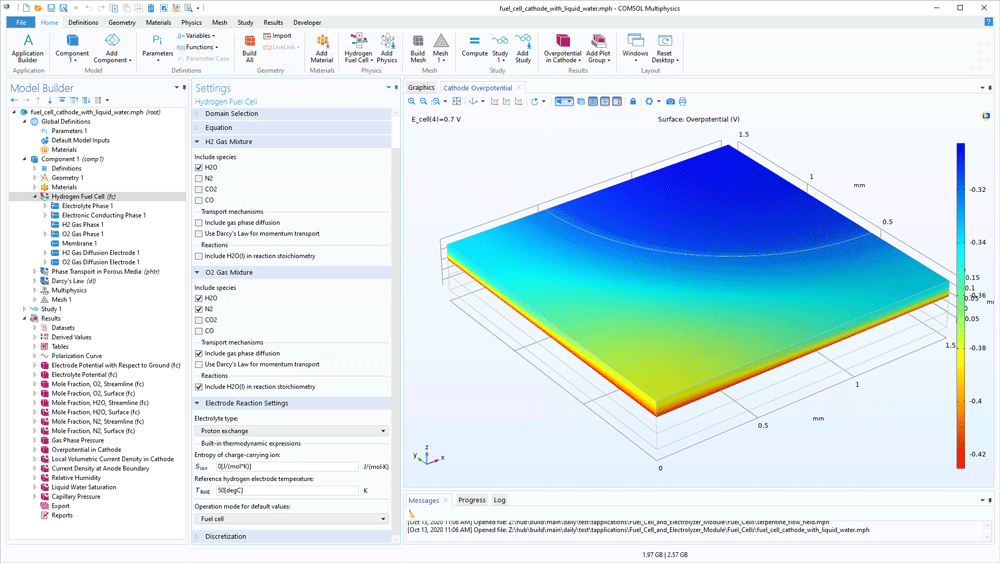
The content of the gas mixtures in the hydrogen and oxygen electrodes may vary for different processes and different operating conditions. The Fuel Cell & Electrolyser Module contains a built-in thermodynamic properties database for hydrogen mixtures and oxygen mixtures. The hydrogen mixture may contain nitrogen, water, carbon dioxide, and carbon monoxide as additional species in order to model byproducts from reforming reactions in addition to hydrogen. The same additional species are available for the oxygen mixture. When the composition is selected and the reference partial pressures are defined, the software can compute the equilibrium electrode potential for the hydrogen and oxygen electrode reactions, thereby also the equilibrium potential for the cell.
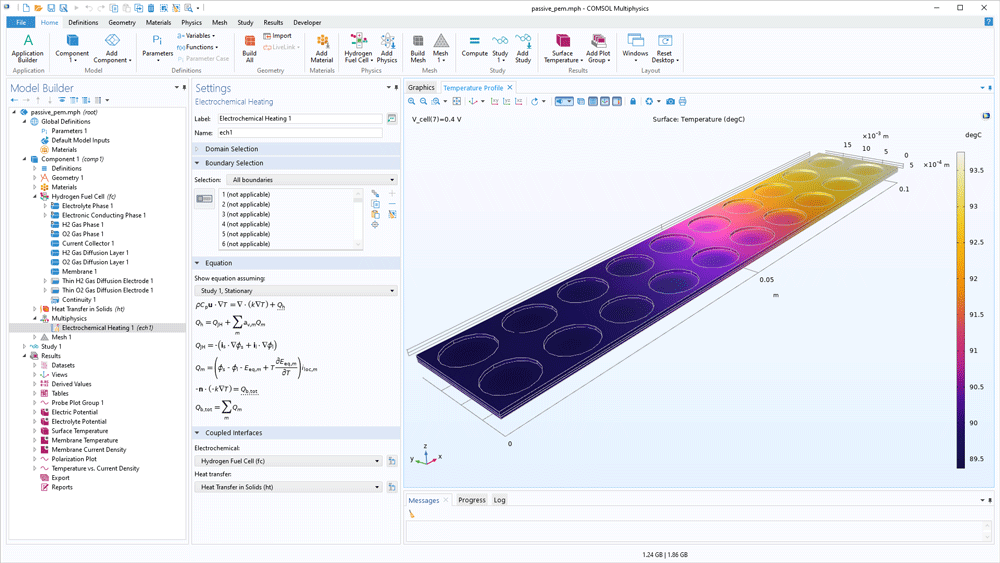
The definition of the energy balance is built-in when using the Fuel Cell & Electrolyser Module. Heat sources and sinks originating from electrochemical reactions, transport of ions and chemical species, as well as current conduction can be added automatically in a heat transfer analysis. Additionally, the thermodynamic database makes it easy to obtain input data for the simulations of thermal management of hydrogen-oxygen cells.
In order to fully evaluate whether or not the COMSOL Multiphysics® software will meet your requirements, you need to contact us. By talking to one of our sales representatives, you will get personalised recommendations and fully documented examples to help you get the most out of your evaluation and guide you to choose the best license option to suit your needs.
Fill in your contact details and any specific comments or questions, and submit. You will receive a response from a sales representative within one business day.